The ABG test (arterial blood gases) is analyzed by taking a sample of arterial blood from the patient’s artery and examining the gases present in it. By 6 easy steps of ABG analysis. The patient’s disease is diagnosed based on the oxygen and carbon dioxide present in the arterial blood. The ABG test sample is taken in a heparinized syringe. The radial artery is primarily used for sampling. The Allen test is performed before the sample is taken, which measures the tolerance of the radial and ulnar arteries.
The ABG tests mainly detect four conditions: respiratory acidosis, respiratory alkalosis, metabolic acidosis, and metabolic alkalosis.
- Respiratory acidosis – caused by the retention of CO2 in the blood and tissue due to hypoventilation.
- Respiratory alkalosis – due to hyperventilation.
- Metabolic acidosis – occurs mainly in conditions of severe diarrhea or DKA and excessive use of aspirin.
- Metabolic alkalosis – due to excessive vomiting, use of diuretics, and gastric aspiration
Nurses play an important role in the care of patients in the hospital. Because of this, there is a need to understand all the tests of that patient, one of them is the ABG test. Several concepts must be taken into account when analyzing an ABG test, which may not be possible for all care providers. Trying to remember many random rules and lack of a standardized approach to ABG. Also, nurses often try to analyze multiple components of the ABG simultaneously. The result is usually confusion and misdiagnosis.
Keeping this in mind, some steps have been prescribed for ABG analysis, which is divided into 6 parts, in other words, we can say Six Easy Steps to ABG Analysis. was developed to provide nurses with an accurate and systematic method of easily interpreting arterial blood gases.
The “6 Easy Steps to ABG Analysis” are listed below for easy reference and will be explained in more detail in the sections that follow. Only we have remembered The 6 Easy Steps to Analysis ABG.
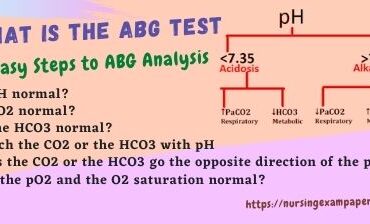
6 Easy Steps to ABG Analysis
- Is pH normal?
- Is CO2 normal?
- Is the HCO3 normal?
- Match the CO2 or the HCO3 with pH
- Does the CO2 or the HCO3 go in the opposite direction of the pH?
- Are the pO2 and the O2 saturation normal?
For our analysis to be effective, notes will have to be written next to the results on our lab slip. Alternatively, the ABG results can be transcribed onto another paper for analysis (see example one below for the format).
Step 1: Analyze the pH
The first step in analyzing ABG is to look at the pH. The pH level in normal blood is 7.35 to 7.45, its average value is 7.40. If the blood pH value falls below 7.35 then it is acidic, and if the pH of the blood goes above 7.45 then it becomes alkaline. If it falls in the normal range, label which side of the 7.4 it falls on. Less than 7.4 is labeled normal/acidic, and greater than 7.4 is labeled normal/alkaline.
Step2: Analyze the CO2
The second step is to examine the pCO2. Normal pCO2 levels are 35-45mmHg. Below 35 is alkaline, and above 45 is acidic. label it.
Step 3: Analyze the HCO3
The third step is to look at the HCO3 level. A normal HCO3 level is 21-27 mEq/L. If the HCO3 is below 21, the patient is acidotic. If the HCO3 is above 27, the patient is alkaline. also, Label it.
Step 4: Match the CO2 or the HCO3 with the pH
Next match either the pCO2 or the HCO3 with the pH to determine the acid-base disorder. For example, if the pH is acidic, and the CO2 is acidic, then the acid-base disturbance is being caused by the respiratory system. Therefore, we call it respiratory acidosis. However, if the pH is acidic and the HCO3 is also acidic, then the acid-base disturbance is caused by the metabolic (or renal) system. Therefore, it will be metabolic acidosis.
Practice Questions For Respiratory System
Step 5: Does the CO2 or HCO3 go in the opposite direction of the pH?
Fifth, does either the CO₂ or HCO3 go in the opposite direction of the pH? If so, there is compensation by that system. For example, the pH is acidic, the CO₂ is acidic, and the HCO3 is alkalotic. The CO₂ matches the pH making the primary acid-base disorder respiratory acidosis. The HCO3 is the opposite of the pH and would be evidence of compensation from the metabolic system.
Step 6: Analyze the pO₂ and O₂ saturation.
Finally, evaluate the PaO2 and O2 sat. If they are below normal there is evidence of hypoxemia. Normal Values (At sea level) Range:-
| Normal Values | Range |
|---|---|
| pH | 7.35-7.45 |
| pCO₂ | 35-45 mmHg |
| pO₂ | 80-100 mmHg |
| O2 Saturation | 94-100% |
| HCO3 | 21-27 mEq/L |
| Base Excess | + or – 3 |
LOOK AT THE CHART BELOW TO DETERMINE THE EVALUATION OF ABNORMAL VALUES:
| pH | PaCO2 | HCO3 | |
| Respiratory Acidosis | ⇩ | ⇧ | ⇩/ N |
| Respiratory Alkalosis | ⇧ | ⇩ | ⇧/ N |
| Metabolic Acidosis | ⇩ | ⇩ / N | ⇩ |
| Metabolic Alkalosis | ⇧ | ⇧ / N | ⇧ |
Notice that if the pH is lower than 7.35 it indicates acidosis, if the pH is higher than 7.45 it indicates alkalosis. The HCO3 is also acidotic if it is low: less than 21 indicates acidosis. If the HCO3 is higher than 27 mEq/L it indicates alkalosis. However, if the CO2 is lower than 35 mmHg it indicates alkalosis, and if the CO2 is higher than 45 mmHg it indicates acidosis. One way to remember this relationship is to use the acronym ROME(Respiratory Opposite Metabolic Equal).
Anion Gap Formula
AG = (Na+ + k+)- (Cl- +HCO3-) (Major Cations – Major Anions)Normal = 10-15 mEq/L
The CO2 is the respiratory component of the ABG, and if it is low and the pH is high the patient would have respiratory alkalosis. They move in opposite directions to match.
The HCO3 is the metabolic component of the ABG. If the HCO3 is low and the pH is low the patient would have metabolic acidosis. They move in the same direction to match.
STEP 5 REFERS TO COMPENSATION. Compensation is the attempt by the body to maintain homeostasis by correcting the pH. The opposite system will do this.
A component of the respiratory system that balances the pH is the dissolved carbon dioxide (CO2) that is produced by cellular processes and removed by the lungs.
The component of the renal system that balances the pH is the dissolved bicarbonate (HCO3) produced by the kidneys. The kidneys also help control pH by eliminating hydrogen (H+) ions. The way the two systems interact is through the formation of carbonic acid (H2CO3). Movement through the carbonic acid system is fluid and constant. What this means is that water (H2O) can combine with CO2 and form carbonic acid. If necessary, carbonic acid (H2CO3) can then break up to form hydrogen ions (H+) and bicarbonate (HCO3). This system works in both directions. By balancing back and forth, a normal pH is achieved.
The respiratory system balances the pH by increasing or decreasing the respiratory rate, thereby manipulating the CO2 level. Fast and deep breathing “blows off” CO2. Conversely, slow and shallow breathing “retains” CO2.
The renal system balances pH by producing HCO3 or by eliminating hydrogen ions (H+). The renal system will reflect changes in metabolic activity within the body. For example, a patient in shock will undergo anaerobic metabolism, which produces lactic acid. The production of lactic acid will bind or use up available HCO3 and will be manifested by a decrease in the HCO3 level. Therefore, the HCO3 level is an indicator of metabolic acid-base balance.
The opposing system must achieve balance. Our body regulates pH by using the opposing system to balance pH. So if the pH is out of balance because of a respiratory disorder, it will be the renal system that makes the corrections to balance the pH. Conversely, if the renal system is to blame for the pH disorder, the respiratory system will have to compensate. This process is called compensation.
Compensation may not always be complete. Complete compensation returns the pH balance to normal. There are times when the imbalance is too large for compensation to restore the pH to normal. This is called partial compensation. Like the seesaw, compensation must come from the opposite system. Step 5 looks to analyze compensation by looking for the system that is going in the opposite direction of the pH.
Now see the Examples of how to apply the 6 Easy Steps to ABG Analysis
EXAMPLE: 1
pH 7.27 acidotic, CO2 53 acidotic, pO2 50 low, O2 sat. 79% low, HCO3 24 normal
6 Step to analysis
- 1: The pH is less than 7.35, therefore it is acidotic.
- 2: The CO2 is greater than 45 and is therefore acidotic.
- 3: The HCO3 is normal.
- 4: The CO2 matches the pH because they are both acidic. Therefore the imbalance is respiratory acidosis. It is acidotic because the pH is acidotic, it is respiratory because the CO2 matches the pH.
- 5: The HCO3 is normal, therefore there is no compensation. If the HCO3 is alkalotic (opposite direction) then compensation would be present.
- 6: Lastly, the PaO2 and O2 sat are low indicating hypoxemia.
The full diagnosis for this blood gas is Uncompensated respiratory acidosis with hypoxemia.⇛ This patient has an acute respiratory disorder.
EXAMPLE: 2
pH 7.55 alkalotic, CO2 27 alkalotic, pO2 99 normals, O2 sat. 98% normal, HCO3 22 normal
6 Step to analysis
- 1: The pH is greater than 7.45, therefore it is alkalotic.
- 2: The CO2 is less than 35 and is therefore alkalotic.
- 3: The HCO3 is normal.
- 4: The CO2 matches the pH because they are both alkalotic. Therefore the imbalance is respiratory alkalosis. It is alkalotic because the pH is alkalotic; it is respiratory because the CO2 matches the pH.
- 5: The HCO3 is normal, therefore there is no compensation. If the HCO3 is acidotic (opposite direction) then compensation would be present.
- 6: Lastly, the PaO2 and O2 sat are normal indicating normal oxygenation.
The full diagnosis for this blood gas is Uncompensated respiratory alkalosis. ⇛ This patient is probably hyperventilating.
list the normal arterial blood gas values, hco3 normal range mmol/l, arterial blood gas calculator, sao2 normal range, normal blood pH range, normal co2, co2 normal range, ABG calculator with base excess, ABG interpretation practice, ABG interpretation chart, ABG interpretation made easy, arterial blood gas interpretation, acute respiratory alkalosis.
Anion Gap Formula
AG = (Na+ + k+)- (Cl- +HCO3-) (Major Cations – Major Anions)Normal = 10-15 mEq/L
Related FAQ On ABG TEST
What is the ABG?
An arterial blood gas test that measures the amount of arterial blood gas such as oxygen and carbon dioxide. The ABG test requires that a small amount of blood is drawn from the radial with a syringe and a thin needle, Some times other sites are also used for ABG Sampling.
What is ABG Test normal range?
The normal range of ABG (pH – 7.35 to 7.45, pCO2 – 35 mmHg To 7.45 mmHg, HCO3 – 21mEq/L- 27 mEq/L, PO2 – 80 – 100 mmHg, O2 Saturetion- 94%- 100%, and Base Excess – +or – 3)
What size needle is used for ABG sampling?
In standard practice, 22 to 26 gauge needles are used for ABG sampling. Because the use of a small gauge needle in ABG sampling can reduce the pain caused to the patient.
Why is the radial artery used for ABG?
The radial artery is the preferred site for arterial puncture/sampling and cannulation. Because there are some reasons for this. the first reason is the easy identification of this artery because of its anatomical position, and the second is the collateral nature of the arterial blood supply provided by the radial and ulnar arteries.
What are the 6 ABG analysis steps?
1. Is pH normal?
2. Is CO2 normal?
3. Is HCO3 normal?
4. Match the CO2 or HCO3 with pH?
5. Does the CO2 or the HCO3 go the opposite direction of the pH?
6. Are the pO2 and the O2 saturation normal?
Thanks for reading. if you have any query please comment below. we answer your query as fast as we can.








One thought on “What is the ABG test, 6 Easy Steps to ABG Analysis”