In this article, we are providing RUHS BSc Nursing Entrance Exam Paper 2019. There are 100 questions in this paper. In which Physics, Chemistry, and Biology subjects have been included. Which follows the syllabus of the 11th and 12th standards. Students who want to take admission in B.Sc Nursing must read the previous papers so that they will get the idea, what kind of questions are asked. So that they will be able to write the exam with more confidence. BSc nursing previous year paper.
RUHS BSc nursing previous year question papers
Q. No. 1. —– cells of the stomach’s gastric pits secrete Hydrochloric acid and intrinsic factor
A. Peptic cells
B. Parietal cells ✅
C. Chief cells
D. Goblet cells
Q. No. 2. During the Chloride shift, the electrical neutrality of RBC is maintained by
A. Diffusion of Cl¯ from plasma to erythrocyte ✅
B. Active transport of k+ from erythrocyte
C. Diffusion of H+ ion from plasma to erythrocyte
D. none
Q. No. 3. Eosinophils
A. has 2-7 lobed
B. Are responsible for protection against infection
C. Are significant in allergic reaction ✅
D. Play important role in detoxification
Q. No. 4. Henle’s loop is meant for the absorption of
A. Potassium
B. Glucose
C. Urea
D. Na+ Na+ ✅
Q. No. 5. The ciliated columnar epithelial cells in humans are known to occur in
A. Bronchioles and Fallopian tubes ✅
B. Eustachian tube and stomach lining
C. Fallopian tubes and urethra
D. Bile duct and esophagus
Q. No. 6. The permeability of the plasma membrane in a resting neuron and muscle fiber is
A. Greater for Na+ than K+
B. Greater for K+ than Na+ ✅
C. Impermeable to K+
D. Impermeable to both the ion
Q. No. 7. Which of the following is false?
A. The endoderm, mesoderm, ectoderm are germ layers.
B. The trophoblast is a germ layer ✅
C. The inner cell mass is a source of embryonic stem cells
D. The blastula is often a hollow ball of cells
Q. No. 8. What is the correct sequence of sperm formation?
A. Spermatid, spermatocyte, spermatogonia, spermatozoa
B. Spermatogonia, spermatocyte, spermatozoa, Spermatid
C. Spermatogonia, spermatozoa, spermatocyte, spermatid
D. Spermatogonia, spermatocyte, spermatid, spermatozoa ✅
Q. No. 9. Menstrual flow occurs due to
A. Progesterone✅
B. FSH
C. Oxytocin
D. Vasopressin
Q. No. 10. Which one of the following is not the function of the placenta? It
A. Facilitates supply of oxygen and nutrients to the embryo
B. Secretes estrogen
C. Facilitates removal of carbon dioxide and waste material from the embryo
D. Secretes oxytocin during parturition ✅
BSc nursing previous year question paper
Q. No. 11. The movement of genes from one linkage group to another is called:
A. Inversion
B. Duplication
C. Translocation
D. Crossing over ✅
Q. No. 12. Alleles are:
A. Different phenotype
B. True breeding homozygous
C. Different molecular forms of the gene
D. Heterozygotes ✅
Q. No. 13. HIV that causes AIDS first starts destroying:
A. B-lymphocytes ✅
B. Leucocytes
C. Helper T-Lymphocytes
D. Thrombocytes
Q. No. 14. There are twelve pairs of ribs out of which —- are directly attached to the sternum
A. First 11 pair
B. First 9 pair
C. First 5 pair
D. First 7 pairs ✅
Q. No. 15. According to the amount and distribution of yolk in an egg, the eggs of amphibians are
A. alecithal and centrolecithal
B. microlecithal and telolecithal
C. mesolecithal and telolecithal ✅
D. mesolecithal and isolecithal
Q. No. 16. Sense organs concerned with equilibrium are
A. Eyes
B. Medulla oblongata
C. Internal ear ✅
D. Nasal chamber
Q. No. 17. The rapid decline in population due to the high mortality rate
A. Population density
B. population Crash ✅
C. population explosion
D. All of the above
Q. No. 18. Among honey bees workers are (BSc Nursing Entrance Exam Paper)
A. Male ✅
B. Female
C. Both male and female
D. Hermaphrodite
Q. No. 19. Plasmid DNA is
A. Extranuclear gene of bacterial cells
B. Best vector DNA for R DNA technology
C. Working as endosymbiont in bacterial cell
D. All of the above ✅
Q. No. 20. Which of the following is/are used as green manures?
A. Crotolariajuncea
B. Melilolusparviflora
C. Trifolium
D. All of the above ✅
Q. No. 21. Natural insecticide obtained from plants
A. Azardiracta
B. Ratenone
C. Pyrethrum and cinerin
D. All of the above ✅
Q. No. 22. Plants which accumulate hydrocarbons of high molecular weight are known as
A. Biogas plant
B. Petro plant ✅
C. Biofertilizer plant
D. None of the above
Q. No. 23. In which climate shifting cultivation takes place
A. Tundra
B. Equatorial ✅
C. Cool temperature
D. Tropical climate
Q. No. 24. Which one of the following is/are medicinal plants commonly found in Rajasthan?
A. Capparisdecidera
B. Prosopiscinearia
C. Tecomella undulate
D. All of the above ✅
Q. No. 25. Nucellus polyembryony if found in
A. Orange
B. Lemon
C. Mango
D. All of the above ✅
BSc nursing entrance exam question paper with answar
Q. No. 26. Who discovered the apomixis in the plant
A. Oswald Tippo
B. Winkler ✅
C. Schwann
D. Robert Hook
Q. No. 27. How many types of soil water are found?
A. one
B. two
C. Three
D. Four ✅
Q. No. 28. Maximum water absorption occurs through which process
A. Osmotic absorption
B. Active absorption
C. Through Suction
D. Passive absorption ✅
Q. No. 29. What happens to the water absorption if the concentration of oxygen is increased in the roots?
A. It stops
B. Increased ✅
C. Decreased
D. Have no effect
Q. No. 30. The process of water absorption follows the following pathway
A. Root hairs, cortex, endodermis, xylem ✅
B. Root hairs, endodermis, cortex, xylem
C. Root hairs, cortex, endodermis, phloem
D. Root hairs, cortex, phloem, xylem
Q. No. 31. The growth of the pollen tube occurs in its
A. Middle part
B. Apical part ✅
C. Posterior part
D. All of the above
Q. No. 32. ……….is formed by the fusion of male gamete and secondary nucleus
A. Zygote
B. Endosperm nucleus ✅
C. Embryosac nucleus
D. Antipodal cells
Q. No. 33. The optimum temperature required for maximum water absorption
A. between 30 ℃ to 45 ℃
B. below 10 ℃
C. between 20 ℃ to 35 ℃ ✅
D. between 10 ℃ to 20 ℃
Q. No. 34. Which one does not occur during Cyclic photophosphorylation?
A. production of oxygen
B. Synthesis of NADP.H2
C. use of H2O ✅
D. All of the Above
Q. No. 35. The mass of a unit cell of CsCl Corresponds to
A. 1 Cs+ and 1 Cl¯ ✅
B. 1 Cs+ and 6 Cl¯
C. 4 Cs+ and 4 Cl¯
D. 8 Cs+ and 1 Cl¯
Q. No. 36. What fraction of the volume of the unit cell is occupied by copper when it crystallizes as FCC?
A. 100 %
B. 74 % ✅
C. 68 %
D. 52.4 %
Q. No. 37. Which of the following changes in thermodynamic quantities represent the ideal solution?
A. △V = 0, △H = 0, △S = +ve, △G = -ve ✅
B. △V = +ve, △H = -ve, △S = -ve, △G = +ve
C. △V = 0, △H = -ve, △S = +ve, △G = 0
D. △V = 0, △H = -ve, △S = +ve, △G = 0
Q. No. 38. The boiling point of C6H6, CH3OH, C6H5NH2, and C6H5NO2 are 80℃, 65℃, 184℃ and 212℃ respectively, which will show the highest vapor pressure at room temperature:
A. C6H6
B. CH3OH ✅
C. C6H5NH2
D. C6H5NO2
Q. No. 39. Exactly 1.00 gram of urea dissolved in 75.0 gram of water gives a solution that boils at 100.114℃. The molecular weight of urea is 60.1. What is Kb for water? (BSc Nursing Entrance Exam Paper)
A. 0.114
B. 100
C. 0.513 ✅
D. 0.222
Q. No. 40. For the reaction,
C2H5I + OH → C2H5OH +I¯
K= 5.03 ×10¯² sec¯¹ at 298 °K and K = 6.71 sec¯¹ at 333 °K
What is the activation energy of the reaction?
A. 2.12 kcal
B. 212 kcal
C. 21.2 kcal ✅
D. 21200 kcal
Q. No. 41. Which of the following statement is correct?
A. The order of a reaction is equal to the sum of the stoichiometric coefficient of the reactant.
B. Order of a reaction can be determined both theoretically and experimentally
C. The order of the reaction is the sum of the exponents of reactants in the rate law equation. ✅
D. Molecularity of a reaction could be both fractional and whole numbers.
Q. No. 42. The volume of a colloidal particle Vc, volume of a solute particle in a true solution Vt, the volume of suspension particle Vs can be arranged
A. Vc = Vt = Vs
B. Vs < Vc < Vt
C. Vs > Vc > Vt ✅
D. Vc > Vs > Vt
Q. No. 43. Tyndal effect would be observed in a
A. True Solution
B. Pure Solvent
C. Precipitate
D. Colloidal solution ✅
Q. No. 44. The ability of an ion to bring about coagulation of a given colloid depends upon
A. The size only
B. The sign of charge alone
C. The magnitude of the charge alone
D. Both magnitude and sign of the charge ✅
Q. No. 45. The voltage of a galvanic cell depends on
A. Concentration
B. Temperature
C. Number of electrons transferred
D. All of them ✅
Q. No. 46. The △E° for the reaction Fe + Zn ++ = Zn + Fe ++ is -0.32 volt. What is the equilibrium concentration of Fe ++ reached when a piece of iron is placed in a 1 M Zn++ solution?
A. 1 M
B. 1.4 M
C. 1.4 x 10 -11 M ✅
D. 1 x 10 -11 M
Q. No. 47. In comparison to other alkaline earth metal Beryllium, oxides are
A. More acidic and is amphoteric ✅
B. More basic and is amphoteric
C. Strong base
D. Neutral
Q. No. 48. Alkali metal oxides are
A. Strong acid
B. Strong base ✅
C. Weak acid
D. Weak base
Q. No. 49. Which the correct relation of atomic radius
A. Li < Mg < Na < Ca < K ✅
B. Li < Mg < Na < K < Ca
C. Li < Na < Mg < Ca < K
D. Li > Mg > Na > Ca > K
Q. No. 50. The bond angle in NO2 +, NO2- and NO2 are related as
A. NO2+ = NO2- = NO2
B. NO2+ > NO2- > NO2
C. NO2+ > NO2 > NO2- ✅
D. NO2+ < NO2 < NO2-
BSc nursing previous year question paper with answer
Q. No. 51. The hybridization of nitrogen in N2F2 is
A. sp
B. sp² ✅
C. sp³
D. dsp²
Q. No. 52. Sulfur may exist in nature as
A. S8
B. S4
C. S2
D. All of the above ✅
Q. No. 53. The shape of the ClF3 molecule is
A. Triangular planar
B. Linear
C. T-shaped ✅
D. Pyramidal
Q. No. 54. The complex ion [CoF6] ¯³ and [CoCN6]+³ are
A. both paramagnetic
B. both diamagnetic
C. paramagnetic and diamagnetic respectively. ✅
D. diamagnetic and paramagnetic respectively.
Q. No. 55. What is the hybridization of the central metal ion in complex ion [CoI4]¯², if the magnetic moment is above 3.5 BM.
A. sp³ ✅
B. sp³d²
C. dsp²
D. d²sp³
Q. No. 56. The correct order of reactivity of SN² reaction of simple alkyl halides is
A. Tertiary > Secondary > Primary > Methyl
B. Methyl > primary > secondary > tertiary ✅
C. Methyl > secondary > primary > tertiary
D. Tertiary > primary > secondary > Methyl
Q. No. 57. Rank the following carbocation in order of increasing stability.
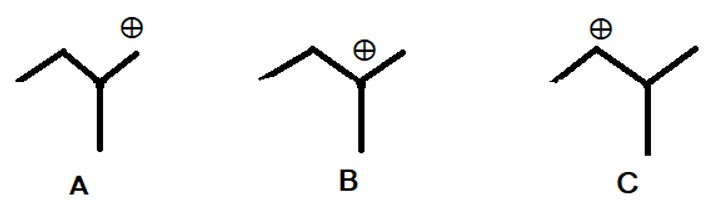
A. A < B < C
B. A < C < B ✅
C. B < A < C
D. C < B < A
Q. No. 58. Which of the following statement is incorrect?
A. polar aprotic solvent favours SN² reaction
B. Polar protic solvent favours SN¯¹ reaction
C. Weak nucleophile favours SN² reaction ✅
D. A relatively unhindered leaving group favours SN² reaction
Q. No. 59. Which of the following is the correct trend of relative acidity?
A. HC ≡ CH > H2C = CH2 > H3C – CH3 ✅
A. HC ≡ CH > H2C = CH2 > H3C – CH3 ✅
B. HC ≡ CH < H2C = CH2 < H3C – CH3
C. H2C = CH2 > HC ≡ CH > H3C – CH3
D. H3C – CH3 > H2C = CH2 < HC ≡ CH
Q. No. 60. A secondary halide with strongly basic nucleophile with heating will preferably give
A. SN¹ product
B. SN² product
C. E¹ product
D. E² product ✅
Q. No. 61. The major product of the reaction between propene and Benzene at 0 °C in presence of strong acid HF is
A. n-propyl benzene
B. isopropyl benzene ✅
C. propenyl benzene
D. No reaction
Q. No. 62. Which of the following substrate does not undergoes readily Friedel Crafts reaction?
A. Toluene
B. Aniline ✅
C. Nitrobenzene
D. Benzoic acid
Q. No. 63. Which of the following electrophilic substitution reaction will give 100 % para product when chlorobenzene undergoes? BSc Nursing Entrance Exam Paper
A. Chlorination
B. Bromination
C. Nitration
D. Sulfonation ✅
Q. No. 64. Which following amino acid contains an aromatic ring in its side chain
A. His
B. Lys
C. Asp
D. Tyr ✅
Q. No. 65. Which of the following amino acid found in proteins will not react with Ninhydrin to give an intense purple-colored anion with the value of 570 nm?
A. His
B. Pro ✅
C. Phe
D. Ile
Q. No. 66. What is the initiation codon for translation?
A. UAA
B. UAG
C. AUG ✅
D. UGA
Q. No. 67. Bakelite is obtained from Phenol when it is reacted with
A. HCHO ✅
B. CH3CHO
C. CH3COCH3
D. (COOH)2
Q. No. 68. Three charges +q, +q, and -2q are placed at the vertices of an equilateral triangle ABC respectively whose side is a. The dipole moment of the system is
A. 3qa
B. 2qa
C. √6qa
D. √3qa ✅
Q. No. 69. Two particles A and B (B is right of A) having charges 8×10¯⁶ C and -2×10¯⁶ C respectively are fixed with a separation of 20 cm. Where should a third charged particle be placed so that it does not experience a net electric force
A. 20 cm left of A
B. 5 cm left of A
C. 20 cm right of B ✅
D. 5 cm right of B
Q. No. 70. An electron of mass m and charge q is accelerated from rest in the uniform electric field of strength E. the velocity acquired by it, as it travels a distance l is
A. (2Eql/m)1/2 ✅
B. (2Eq/lm)1/2
C. (2Em/ql)1/2
D. (Eq/lm)1/2
Q. No. 71. Two charged spheres separated at distance d exert a force F on each other. If they are immersed in a liquid of dielectric constant 2, then what is the force (if all conditions are the same)
A. F/2 ✅
B. F
C. 2F
D. 4F
Q. No. 72. A capacitor of capacity C has charge Q and the stored energy is W. If the charge is increased to 2Q, the stored energy will be
A. 2W
B. W/2
C. 4W ✅
D. W/4
Q. No. 73. Two bulbs of wattage 40 W and 100 W rated at 220 V are connected in series across a 440 V. What will happen
A. 40 W bulb will fuse ✅
B. 100 W bulb will fuse
C. Both bulbs will fuse
D. nothing will happen
Q. No. 74. A charged particle of mass m and charge q describes the circular motion of radius r in a uniform magnetic field of strength B. The frequency of revolution is (BSc Nursing Entrance Exam Paper)
A. Bq/2πm ✅
B. Bq/2πrm
C. 2πM/Bq
D. Bm/2πq
Q. No. 75. A current flows in a conductor from east to west. The direction of the magnetic field at a point above the conductor is
A. towards north ✅
B. towards south
C. towards east
D. towards west
BSc nursing entrance exam previous year question papers with answer pdf
Q. No. 76. The dimensional formula of magnetic induction is
A. [MT⁴A-¹]
B. [MT-²A-¹] ✅
C. [MLA-²]
D. [MT³A]
Q. No. 77. A magnet of magnetic moment M is situated with its axis along the direction of a magnetic field of strength B. The work done in rotating it by an angle of 180° will be
A. -MB
B. +MB
C. zero
D. +2MB ✅
Q. No. 78. What inductance would be needed to store 1 KWh of energy in a coil carrying a 200 A current
A. 1800H
B. 180H ✅
C. 80H
D. 800H
Q. No. 79. Two pure inductors each of self-inductance L are connected in parallel but are well separated from each other. The total inductance is
A. 2L
B. L
C. L/2 ✅
D. L/4
Q. No. 80. The magnetic flux linked with a coil, in Webers, is given by the equation. φ = 3t²+4t+9 Then the magnitude of induced emf at t =2 sec will be
A. 4V
B. 3V
C. 8V
D. 16V ✅
Q. No. 81. The resistance of a coil for DC is 5 Ohm. In the case of AC, the resistance will
A. remain 5 Ohm
B. decrease
C. increase ✅
D. be zero
Q. No. 82. The focal length of the convex lens in air is 10 cm. Its focal length in water will be
A. 30 cm
B. 4 cm
C. 40 cm ✅
D. 10 cm
Q. No. 83. The wavelength of light in a vacuum is 6000 Å. What will be the wavelength after passing through the glass whose refractive index is 1.5?
A. 4000 Å ✅
B. 6000 Å
C. 9000 Å
D. 15000 Å
Q. No. 84. When the light is refracted, which of the following does not change
A. Wavelength
B. Amplitude
C. Velocity
D. Frequency ✅
Q. No. 85. How does the refractive index (μ) of material vary with respect to wavelength (λ), where A and B are constant
A. μ=A+B/λ² ✅
B. μ=A+Bλ²
C. μ=A+B/λ
D. μ=A+Bλ
Q. No. 86. The critical angle of a prism is 30°. The velocity in the medium is
A. 1.5×10⁸m/s ✅
B. 4.5×10⁸ m/s
C. 3×10⁸ m/s
D. 4.5×10⁷ m/s
Q. No. 87. Which of the following is conserved when light waves interfere?
A. Intensity
B. Energy ✅
C. Amplitude
D. Momentum
Q. No. 88. If the two waves represented by y1=4sin ωt and y2 =3sin (ωt+ π/2) interfere at a point, the amplitude of the resulting wave will be about. BSc Nursing Entrance Exam Paper
A. 7
B. 5
C. 6 ✅
D. 3.5
Q. No. 89. In double slits experiments, for a light of which color the fringe width will be minimum
A. green
B. violet ✅
C. red
D. yellow
Q. No. 90. Yellow light is used in single slit diffraction with a slit width of 0.6 mm. If the yellow light is replaced by X-rays, then the observed pattern will reveal
A. that the central maximum is narrower
B. more number of fringes
C. less number of fringes
D. no diffraction pattern ✅
Q. No. 91. The work function of a metal is 1.6×10-¹⁹J. When the metal surface is illuminated by the light of wavelength 6400 Å, then the maximum kinetic energy of emitted photoelectrons will be approximately (h = 6.6×10-³⁴ J/s)
A. 1.4×10-¹⁹ J ✅
B. 2.8×10-¹⁹ J
C. 14×10-¹⁹ J
D. 1.4×10-¹⁹ eV
Q. No. 92. What will be a ratio of de- Broglie wavelength of proton and α – a particle of the same energy
A. 2:1 ✅
B. 1:2
C. 4:1
D. 1:4
Q. No. 93. Which one of the following series of hydrogen spectrum is in the visible region?
A. Lyman
B. Balmer ✅
C. Paschan
D. Bracket
Q. No. 94. Aβ -particle is emitted by radioactivity nucleus at the time of conversion of a
A. neutron into proton ✅
B. proton into neutron
C. nucleons into energy
D. Positron into energy
Q. No. 95. When the electrical conductivity of a semi-conductor is due to the breaking of its covalent bonds, then the semi-conductor is said to be
A. donar
B. acceptor
C. intrinsic ✅
D. extrinsic
Q. No. 96. A p-n junction has a thickness of the order of
A. 1 cm
B. 1mm
C. 10¯⁶ m ✅
D. 10¯¹² cm
Q. No. 97. In forward bias, the width of the potential barrier in a p-n junction diode
A. increase
B. decreases ✅
C. remains same
D. first increase then decreases
Q. No. 98. The transistors provide good power amplification when they are used in
A. Common collector configuration ✅
B. Common emitter configuration
C. Common base configuration
D. none of these
Q. No. 99. A Zener diode is used for
A. Rectification
B. Modulation
C. Detection
D. Voltage regulation ✅
Q. No. 100. The maximum amount of radiation in the earth’s atmosphere is of the type
A. X-rays
B. Y-rays
C. Ultraviolet
D. Infrared ✅
Also, Read –
OT Technician Exam Paper with Answer key
Safdarjung Hospital VMMC Nursing Officer Gr-II Exam paper
RRB Nursing Question Paper with Answers pdf
RRB Question Paper Previous Year








